Get Healthy!
Staying informed is also a great way to stay healthy. Keep up-to-date with all the latest health news here.
30 Jun
Regular Exercise Helps Ease Kids’ Depression and Anxiety
A new study finds exercise decreases symptoms of depression and anxiety in children and teens – and may offer an alternative to antidepressants.
27 Jun
Low Levels of A Common Drinking Water Contaminant Linked to Premature Birth
In a new study, nitrate levels well below the EPA limit for drinking water were associated with an increased risk of premature birth and low-birthweight babies.
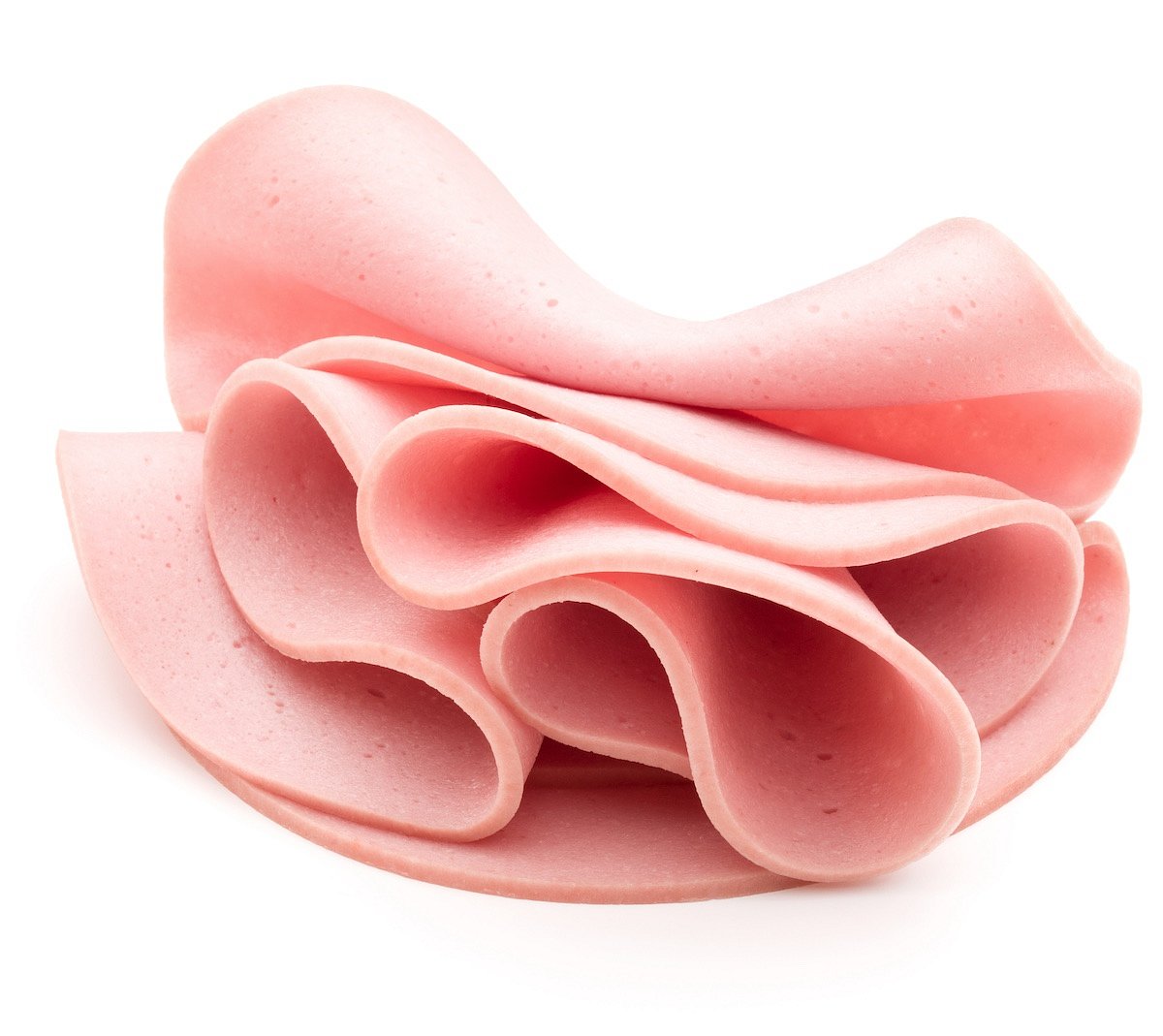
Close to 150,000 Pounds of Ready-to-eat Bologna Recalled Due to Mislabeling
Gaiser's European Style Provisions Inc. is recalling almost 150,000 pounds of ready-to-eat bologna due to mislabeling.
The recalled lunch meats contain meat or poultry source materials that are not listed on the product labels, CBS News reports.
The bologna was sold under various names and labels and was prod...
- Denise Mann HealthDay Reporter
- |
- June 30, 2025
- |
- Full Page
Nestle, Other Food Companies Vow to Ban Artificial Colors
Nestle has joined a growing list of major food companies pledging to voluntarily eliminate artificial colors from their U.S. products by the middle of next year amid mounting health concerns.
"We are always looking for different ways to offer great tasting, compelling choices for our consumers," Nestle's U.S. CEO Marty Thompson said in a s...
- Denise Mann HealthDay Reporter
- |
- June 30, 2025
- |
- Full Page
Need A 'Eureka' Moment? Take A Good Nap, Study Says
Do you enjoy “eureka” moments, when sudden insight or inspiration strikes seemingly from nowhere?
Then you definitely need to sleep on it, a new study says.
People are more likely to have sudden “eureka” moments on nagging problems if they can reach a deeper phase of sleep during a nap, researchers reported Ju...
- Dennis Thompson HealthDay Reporter
- |
- June 30, 2025
- |
- Full Page
Could Your Address Determine Your Dementia Risk?
Your address might influence your risk for dementia, a new study says.
People living in poor neighborhoods appear to be more likely to have biological risk factors for inflammation and Alzheimer’s disease, researchers reported June 25 in the journal Neurology.
“These results suggest that neighborhood disadvantage...
- Dennis Thompson HealthDay Reporter
- |
- June 30, 2025
- |
- Full Page
Exercise Helps Kids' Mood Disorders, Can Serve As Alternative To Meds, Review Finds
Regular exercise can ease mood disorders in children and teens, offering an alternative to medications like antidepressants, a new evidence review has concluded.
Both anxiety and depression decrease when kids take part in structured exercise programs, researchers reported June 26 in Journal of the American Academy of Child & Adoles...
- Dennis Thompson HealthDay Reporter
- |
- June 30, 2025
- |
- Full Page
Severe Bleeding After Delivery Linked To Long-Term Heart Health Problems
The health of women who experience severe bleeding after giving birth can remain in peril for up to 15 years afterward, a major new evidence review says.
Women who survive postpartum hemorrhage are 76% more likely to suffer health problems like heart failure, stroke and heart disease for more than a decade afterward, researchers report in ...
- Dennis Thompson HealthDay Reporter
- |
- June 30, 2025
- |
- Full Page
New Test Can Predict, Help Prevent Miscarriage
An experimental test can predict a woman’s risk of miscarriage, based on problems in the womb lining that occur prior to pregnancy.
In some women, an essential biological process that prepares the endometrium for pregnancy doesn’t progress properly, increasing the risk of miscarriage, researchers report in the journal Scien...
- Dennis Thompson HealthDay Reporter
- |
- June 30, 2025
- |
- Full Page
'Pill-On-A-String' Could Revolutionize Testing For Throat Cancer
The thought of swallowing a pill on a thread isn’t the most pleasant notion, but it could be a vastly better alternative for people at increased risk of throat cancer, a new study says.
For nearly 20 years, U.K. resident Duncan Cook has had regular endoscopies to monitor his case of Barrett’s esophagus, a condition in which aci...
- Dennis Thompson HealthDay Reporter
- |
- June 30, 2025
- |
- Full Page
Toxic Mercury in Gators Is a Warning Sign for Other Species
The swamps of Georgia and South Carolina harbor something more dangerous than the alligators for which they’re best known.
Researchers studying the ancient reptiles found high levels of mercury, a potent neurotoxin. Their discovery — reported in the June issue of the journal Environmental Toxicology and Chemistry &mdas...
- Carole Tanzer Miller HealthDay Reporter
- |
- June 29, 2025
- |
- Full Page
Want To Run Better? Try Focusing Your Eyes Straight Ahead
If you’re looking to boost your running performance, try this simple tip: Keep your eyes on the finish line.
New research shows that narrowing your focus while running — especially as you get closer to the end — can help you run faster and push harder.
The study, which examined nearly 1,600 runners, found that this ...
- I. Edwards HealthDay Reporter
- |
- June 28, 2025
- |
- Full Page
From Transgender Care To Vaping: Key Takeaways From SCOTUS 2025 Term
From allowing states to ban gender-transition care and sales of flavored vapes to minors to rolling back the landmark Clean Air Act, the U.S. Supreme Court had a consequential term.
The Washington Post cites these as among the high court’s most consequential decisions of 2025:
Birthright citizenship: I...
- Carole Tanzer Miller HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
U.S. Stops Funding for Gavi Global Vaccine Program, Sparking Backlash
The United States will stop sending money to Gavi, a global group that helps vaccinate children in low-income countries, U.S. Health and Human Services Secretary Robert F. Kennedy Jr. said Wednesday.
The decision was made public in a video shared at a Gavi summit in Brussels. In it, Kennedy said Gavi has not done enough to address vaccine ...
- I. Edwards HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
RFK Jr.-Appointed Vaccine Panel Removes Flu Shot Ingredient
A U.S. vaccine advisory panel voted Thursday to stop recommending flu shots that contain thimerosal, a move that experts say may reduce access to vaccines without making them any safer.
The vote came from a newly appointed group of people that now make up the Advisory Committee on Immunization Practices (ACIP), led by U.S. Health and Human...
- I. Edwards HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
COVID Vaccine Labels To Warn of Rare Heart Risk
The U.S. Food and Drug Administration (FDA) has added new warnings to the Pfizer and Moderna COVID-19 vaccines about a rare heart condition that mostly affects young men.
The update expands earlier warnings about myocarditis, a type of heart inflammation, The Associated Press reported.
The condition is still very rare ...
- I. Edwards HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
Everyday Packaging May Shed Tiny Plastics Into Your Food, Study Finds
Opening a bottle or unwrapping a piece of deli meat could be adding tiny plastic particles to your food, new research reveals.
Microplastics and nanoplastics can enter food during packaging, processing and even normal use, like twisting a bottle cap or tearing off a plastic wrapper, according to a study published June 25 in NPJ Science...
- I. Edwards HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
U.S. Gun Suicides Continue Record Rise
Gun-related suicides in the U.S. reached record highs for the third straight year in 2023, a new report on gun violence says.
About 27,300 gun-related deaths — 58% of all gun deaths — were suicides in 2023, according to research from Johns Hopkins Bloomberg School of Public Health.
That means an American used a gun to kil...
- Dennis Thompson HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
Fasting Might Not Be Necessary Prior To Surgery, Review Concludes
Fasting is a well-known hassle associated with surgery.
Patients are required to go without food or liquid for hours because of fears they’ll vomit while under anesthesia, potentially causing pneumonia if stomach contents are inhaled.
But this long-standing practice might not be necessary, a new evidence review says.
Ther...
- Dennis Thompson HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
Many Kids Unnecessarily Hospitalized Following Allergic Reactions
Many kids are spending a lot of unnecessary time under observation in a hospital following a sudden allergic emergency, a new study concludes.
About 17% of kids are admitted for overnight observation following a scary allergic reaction to food, medicine or insect bites, researchers reported.
But 95% of children treated for alle...
- Dennis Thompson HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
High-Fiber Diet Reduces Risk of Hardened Arteries
Noshing on veggies, grains, beans and other high-fiber foods can help your heart as well as your gut health, a new study says.
People with low-fiber diets are more likely to have narrowed arteries caused by the buildup of plaque, researchers reported recently in the journal Cardiovascular Research.
CT scans also revealed tha...
- Dennis Thompson HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page
Body Fat Analysis, Waist Size Better Than BMI For Assessing Health
A person’s body fat percentage provides a better estimate of their risk for early death than their body mass index (BMI), a new study says.
People with a high body-fat percentage were 78% more likely to die within 15 years from any cause and 3.6 times more likely to die from heart disease, researchers reported June 24 in the Anna...
- Dennis Thompson HealthDay Reporter
- |
- June 27, 2025
- |
- Full Page