Get Healthy!
505 Results for search "Food &, Drug Administration".
Health News Results - 505
A new gene therapy for sickle cell disease was deemed safe by a U.S. Food and Drug Administration advisory panel on Tuesday, paving the way for full approval by early December.
The FDA had already decided that the therapy, known as exa-cel, was effective.
Developed by Vertex Pharmaceuticals of Boston and CRISPR Therapeutics of Switzerland, exa-cel frees patients from the excruciatin...
- Robin Foster and Ernie Mundell HealthDay Reporters
- |
- November 1, 2023
- |
- Full Page
Certain pureed fruit pouches from WanaBana may contain dangerous levels of lead, the U.S. Food and Drug Administration has warned.
Parents and caregivers should not buy or serve WanaBana apple cinnamon fruit puree pouches to children, the agency said in a recent
Federal regulators are warning consumers to stop using eyedrops and gels from several major brands after finding unsanitary conditions in a manufacturing plant.
Twenty-six eye care products are part of the alert. Contaminated products have the potential to cause eye infection and blindness because drugs applied to the eye can bypass some of the body's defenses.
Those concerning the ...
- Cara Murez HealthDay Reporter
- |
- October 30, 2023
- |
- Full Page
Patients with sickle cell disease may soon have two new treatments to try.
On Tuesday, a U.S. Food and Drug Administration advisory committee will weigh the merits of a new gene therapy for the painful, inherited condition, which typically strikes Black people.
The agency is expected to make a decision on that therapy in early December, and it also plans to decide on a second new tr...
- Cara Murez HealthDay Reporter
- |
- October 30, 2023
- |
- Full Page
The U.S. Food and Drug Administration has proposed a ban on the use of formaldehyde in hair relaxers over concerns about its link to respiratory problems and certain cancers.
Right now, the FDA only discourages u...
- Cara Murez HealthDay Reporter
- |
- October 18, 2023
- |
- Full Page
A proposed rule from federal regulators that would ban menthol cigarettes and flavored cigars has been sent to the White House Office of Management and Budget for final review.
- Cara Murez HealthDay Reporter
- |
- October 17, 2023
- |
- Full Page
The U.S. Food and Drug Administration is warning consumers about risks of using compounded versions of the drug ketamine, often taken for psychiatric disorders.
Compounded products are not evaluated by the FDA for safety and effectiveness. They're also not regulated like approved drugs, so they present a greater risk.
“Although compounded drugs can serve an important medical need ...
- Cara Murez HealthDay Reporter
- |
- October 11, 2023
- |
- Full Page
Cigarette makers are using synthetic menthol substitutes in what appears to be an effort to skirt a looming federal menthol ban, researchers say.
The menthol flavor appeals to younger and newer smokers, according to investigators at Duke Health in Durham, N.C., and Yale University in New Haven, Conn.
These new “non-menthol” cigarettes are being introduced in states that have alr...
- Cara Murez HealthDay Reporter
- |
- October 11, 2023
- |
- Full Page
Faced with growing reports of inaccurate clinical lab tests, the U.S. Food and Drug Administration on Friday announced that it will for the first time regulate these vital diagnostic tools.
Many Americans might have assumed that the FDA already had oversight of all medical tests; it does not.
However, FDA Commissioner
An advisory panel to the U.S. Food and Drug Administration on Wednesday voted resoundingly against recommending a stem cell-based experimental treatment for ALS.
Although the FDA isn't bound by the votes of its advisory panels, agency scientists have already penned a scathing review of the drug, called NuOwn.
Th...
- Cara Murez HealthDay Reporter
- |
- September 28, 2023
- |
- Full Page
Ozempic, a type 2 diabetes drug that has increasingly been used to help with weight loss, will now be labeled as having the potential to block intestines.
The U.S. Food and Drug Administration recently made the label update for the drug made by ...
- Cara Murez HealthDay Reporter
- |
- September 27, 2023
- |
- Full Page
A Pfizer plant that makes vital drugs, anesthesia and hospital supplies has restarted production after a 10-week shutdown.
The plant, located in Rocky Mount, N.C., sustained severe tornado damage on July 19, when roofs were ripped off and medications tossed around.
"This expedited restart is a proud achievement for the Rocky Mount team; however, it is only the first step toward ful...
- Cara Murez HealthDay Reporter
- |
- September 26, 2023
- |
- Full Page
Kraft Heinz said it is recalling over 83,000 packs of its Kraft Singles American processed cheese slices because of a packaging defect in the plastic that wraps the cheese slices.
A temporary issue developed on one of the wrapping machines, making it possible for a thin strip of individual film to stay on the cheese slice after the wrapper is removed. Having this film on the cheese could ...
- Cara Murez HealthDay Reporter
- |
- September 22, 2023
- |
- Full Page
A new government report finds that federal regulators need to do more to help in the battle to keep kids and teens off tobacco.
Among the report's findings were that the U.S. Food and Drug Administration needs to get tough on retailers selling tobacco to youth and should improve its oversight of online retailers.
The FDA should also work with the Bureau of Alcohol, Tobacco, Firearm...
- Cara Murez HealthDay Reporter
- |
- September 21, 2023
- |
- Full Page
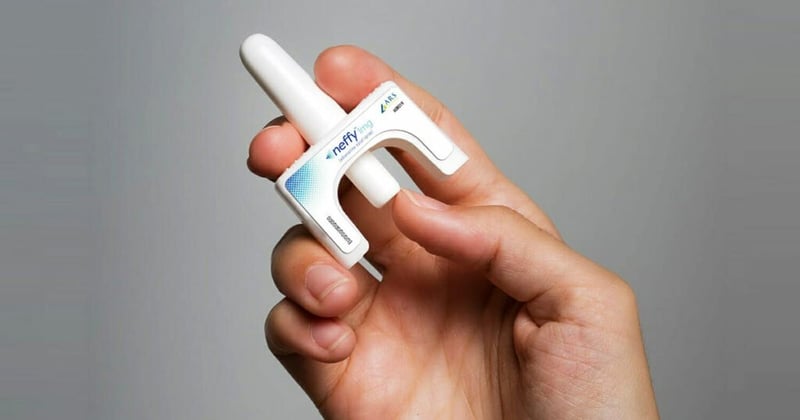
In a surprising move, the U.S. Food and Drug Administration (FDA) has opted not to approve a needle-free alternative to the EpiPen for emergency treatment of severe allergic reactions.
Approval of the Neffy nasal spray was widely anticipated. An FDA advisory panel voted to recommend approval of the drug for children and adults in May. While the FDA is not obligated to follow the advice of...
- Denise Mann HealthDay Reporter
- |
- September 20, 2023
- |
- Full Page
Another diabetes drug maker is taking legal action against businesses in several states, alleging that they're “fraudulently claiming” that their compounded products are the same as its medication.
This time, it's Eli Lilly suing certain medical spas, wellness centers and compounding pharmacies over its medication Mounjaro.
Mounjaro contains the active ingredient tirzepatide an...
- Cara Murez HealthDay Reporter
- |
- September 20, 2023
- |
- Full Page
Following on an approval granted Monday by the U.S. Food and Drug Administration, an expert panel from the U.S. Centers for Disease Control and Prevention on Tuesday also signed off on new COVID boosters for Americans.
Final approval is expected from CDC Director Dr. Mandy Cohen, which would set the stage for the updated vaccines to soon become available.
The COVID-19 shots from Pf...
- Robin Foster HealthDay Reporter
- |
- September 12, 2023
- |
- Full Page
For decades, sick people have been taking essentially worthless over-the-counter cold remedies to clear their stuffy noses, a key advisory panel for the U.S. Food and Drug Administration said Tuesday.
The panel voted unanimously that nonprescription oral medications containing phenylephrine -- including Sudafed PE, Vicks Sinex and Benadryl Allergy Plus Congestion -- don't do anything to e...
- Dennis Thompson HealthDay Reporter
- |
- September 12, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Monday gave the green light to new COVID boosters for Americans, setting the stage for the updated vaccines to become available within days.
The COVID-19 shots from Pfizer and Moderna will join the flu shot and newly approved RSV shots as part of a three-pronged public health strategy to tame the spread of all three viruses this coming winter. The ...
- Robin Foster HealthDay Reporter
- |
- September 11, 2023
- |
- Full Page
New COVID-19 booster shots could soon pass the needed hurdles for vaccinations to begin next week.
Sources familiar with U.S. Food and Drug Administration plans say boosters could be approved as soon as Friday, NBC News reported.
The U.S. Centers for Disease Control and Prevention Advisory Committee on Immunization Practices is scheduled to meet on Tuesday. CDC director
- Cara Murez HealthDay Reporter
- |
- September 7, 2023
- |
- Full Page
The U.S. Department of Health and Human Services has asked the U.S. Drug Enforcement Agency to reclassify marijuana as a less dangerous drug under the Controlled Substances Act, a move that could potentially expand acceptance of the drug.
The DEA confirmed receiving an Aug. 29 letter requesting the change and will begin its own review, a spokesperson told Bloomberg News.
Th...
- Cara Murez HealthDay Reporter
- |
- August 31, 2023
- |
- Full Page
Tainted eye drops are back in the news, with federal regulators warning consumers not to use certain eye drops because of contamination concerns.
- Cara Murez HealthDay Reporter
- |
- August 23, 2023
- |
- Full Page
The U.S. Food and Drug Administration has warned that consumers should not use certain pregnancy, ovulation, urine, UTI and breast milk test kits over concerns that the tests may not be safe and effective.
The tests in question were manufactured by Universal Meditech Inc. (UMI), though they were branded under several names and may not include information about UMI on their packaging, the ...
- Cara Murez HealthDay Reporter
- |
- August 14, 2023
- |
- Full Page
Whether you got a tattoo on a whim or after much thought, that ink on your body is fairly permanent.
Tattoo removal is possible, but it comes with risks, according to the U.S. Food and Drug Administration, which regulates tattoo ink and pigment, as well as the laser devices used to remove them. State and local authorities typically oversee tattooing practices.
The FDA has cleared se...
- Cara Murez HealthDay Reporter
- |
- August 6, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Friday approved a new pill, called zuranolone, that may quickly ease severe postpartum depression and help millions of women regain their emotional equilibrium following childbirth.
Taken as a pill once a day for two weeks, zuranolone (Zurzuvae) showed “rapid, significant and sustained” reductions in depressive symptoms when compared to a place...
- Denise Mann HealthDay Reporter
- |
- August 5, 2023
- |
- Full Page
While demand for prescription stimulants is surging, a shortage of the drugs persists, so federal officials have stepped in and asked drug companies to ramp up production of the medications.
Officials from both the U.S. Food and Drug Administration and the U.S. Drug Enforcement Administration (DEA) made the joint request.
“The FDA and DEA do not manufacture drugs and cannot requir...
- Cara Murez HealthDay Reporter
- |
- August 2, 2023
- |
- Full Page
All eyes are on the U.S. Food and Drug Administration this week as the agency weighs approval of a new pill that may quickly treat and ease severe postpartum depression.
Approval of the drug could help millions of women regain their emotional equilibrium following childbirth. The FDA's decision is expected by Friday.
Taken as a pill once a day for two weeks, zuranolone showed “ra...
- Denise Mann HealthDay Reporter
- |
- August 2, 2023
- |
- Full Page
Eight healthy habits could add years to your life.
A new study of more than 700,000 U.S. veterans breaks down the habits that when adopted by middle age, can help someone live substantially longer than folks who don't have these habits.
These are the big eight:
- Be physically active.
- Don't smoke.
- Don't get addicted to opioids.
- Don't binge-drink on a...
- Cara Murez HealthDay Reporter
- |
- July 24, 2023
- |
- Full Page
Another experimental drug meant to slow the damage of Alzheimer's appears poised to join a growing arsenal of new treatments for this memory-robbing disease.
In research published online Monday in the Journal of the American Medical Association and presented simultaneously at the Alzheimer's A...
- Robin Foster HealthDay Reporter
- |
- July 17, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Thursday approved the nation's first over-the-counter birth control pill, a move that will likely pave the way for far greater access to contraception for Americans.
Women will be able to buy the progestin-only oral contraceptive at drug stores, convenience stores and grocery stores, the FDA said. There is no age limit.
Opill, which is made b...
- Dennis Thompson HealthDay Reporter
- |
- July 13, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Thursday gave full approval to the Alzheimer's drug Leqembi, clearing the way for insurance coverage of the pricey drug.
“The full FDA approval will open the floodgates for people with early Alzheimer's to get this drug. It's a big deal because it's very expensive at $26,500 per year,"
Several companies are selling copycat food items that have the potential to trick people, including children, into consuming dangerous quantities of cannabis.
On Wednesday the U.S. Food and Drug Administration and the Federal Trade Commission warned six companies about selling these copycat food products that contain delta-8 tetrahydrocannabinol, also known as delta-8 THC, a substance fou...
- Cara Murez HealthDay Reporter
- |
- July 5, 2023
- |
- Full Page
A new blood test approved by the U.S. Food and Drug Administration can predict imminent preeclampsia, helping pregnant women who are at risk of this severe and sometimes deadly form of high blood pressure.
The test can identify with 96% accuracy which women with sometimes-vague symptoms will develop preeclampsia within the following two weeks, The New York Times reported this wee...
- Cara Murez HealthDay Reporter
- |
- July 5, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Thursday approved the drug Elevidys, the first gene therapy for the treatment of children with Duchenne muscular dystrophy (DMD).
The groundbreaking treatment will not be cheap: Drugmaker Sarepta Therapeutics Inc. said it would charge $3.2 million for the one-time IV treatment, the Associated Press reported. Like most medicines in the Unit...
- Steven Reinberg HealthDay Reporter
- |
- June 23, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Tuesday approved two drugs that have been used in adults with type 2 diabetes for years for use in children aged 10 and up.
The approvals of Jardiance (empagliflozin) and Synjardy (empagliflozin and metformin hydrochloride) provide a new class of medications for pediatric type 2 diabetes. They join metformin, which has been approved for children wi...
- Cara Murez HealthDay Reporter
- |
- June 21, 2023
- |
- Full Page
The maker of three popular drugs that treat weight loss and diabetes said Tuesday that it has begun legal proceedings against businesses that are selling compounded versions of its products that are not approved by the U.S. Food and Drug Administration.
In a sta...
- Cara Murez HealthDay Reporter
- |
- June 21, 2023
- |
- Full Page
An advisory panel to the U.S. Food and Drug Administration voted unanimously on Thursday to recommend that COVID booster shots be updated for the fall to protect solely against one of the three XBB variants that have taken hold in the United States.
Those three XBB variants, which are all sublineages of the Omicron variant, are XBB.1.5, XBB.1.16 and XBB.2.3, CNN reported. What wi...
- Robin Foster HealthDay Reporter
- |
- June 15, 2023
- |
- Full Page
Regulators want your next tattoo to be as safe as it is trendy.
The U.S. Food and Drug Administration has issued a draft of new guidance aimed at helping manufacturers and distributors of tattoo inks prevent microbial contamination.
“Wit...
- Cara Murez HealthDay Reporter
- |
- June 15, 2023
- |
- Full Page
COVID-19 boosters may be offered this fall, but first scientists need to determine what strains to target and who should receive the shots.
Advisers to the U.S. Food and Drug Administration are slated to meet Thursday to discuss plans for fall, a decision with a deadline because drugmakers will need to have the time to manufacture the shots, NBC News reported.
The process i...
- Cara Murez HealthDay Reporter
- |
- June 14, 2023
- |
- Full Page
With the United States facing a high number of drug shortages, a Chinese company may help to boost the supply of one in particular, the chemotherapy agent cisplatin.
The U.S. Food and Drug Administration is working with the Chinese drugmaker Qilu Pharmaceutical to import the widely used cancer drug. The Canadian pharmaceutical company Apotex will distribute the medication in 50-milli...
- Cara Murez HealthDay Reporter
- |
- June 5, 2023
- |
- Full Page
It's tempting to treat little skin bumps on your own, but that delays proper diagnosis and treatment that may work better, federal regulators cautioned.
Among the many types of skin conditions a person can contract are a virus called molluscum, which look like white, pink or flesh-colored bumps.
Products marketed as treatments for molluscum have not been approved by the U.S. Food an...
- Cara Murez HealthDay Reporter
- |
- June 5, 2023
- |
- Full Page
Older adults may have a second vaccine option for RSV following the U.S. Food and Drug Administration's approval of a Pfizer vaccine on Wednesday.
The other shot for adults 60 and up is made by GSK. It was approved May 3.
Both should be available by fall, before the seasonal spread of respiratory syncytial virus (RSV), The New York Times reported.
The Pfizer ...
- Cara Murez HealthDay Reporter
- |
- June 1, 2023
- |
- Full Page
Americans with COVID-19 have been taking Paxlovid since it was approved under emergency use in late 2021. Today, the U.S. Food and Drug Administration granted full approval to the drug.
This approval will allow drugmaker Pfizer to sell the medication at market rate once government supplies are used up.
Paxlovid is the fourth antiviral drug and first pill approved by the FDA to treat...
- Cara Murez HealthDay Reporter
- |
- May 25, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Monday approved a second nasal spray for reversing an opioid overdose.
To be sold as Opvee, the spray contains the medication nalmefene hydrochloride and will be available to Americans aged 12 and older with a prescription, the FDA said.
"The agency continues to advance the FDA Overdose Prevention Framework and take actionable steps that enc...
- Cara Murez HealthDay Reporter
- |
- May 23, 2023
- |
- Full Page
Patients with Crohn's disease have a new treatment option, following U.S. Food and Drug Administration approval of a pill called Rinvoq (upadacitinib).
Rinvoq is meant to treat adults with moderately to severely active Crohn's disease who have not had success with TNF (tumor necrosis factor) blockers. The daily pill is the first oral treatment for this group of patients.
Crohn's is ...
- Cara Murez HealthDay Reporter
- |
- May 19, 2023
- |
- Full Page
It's important to ask questions when your doctor or dentist writes you a new prescription.
This is especially true for opioid pain medications, such as hydrocodone, oxycodone or morphine.
While these drugs are approved by the U.S. Food and Drug Administration for acute and chronic pain, they can have serious side effects, including addiction and even death.
Misuse of opioids ...
- Cara Murez HealthDay Reporter
- |
- May 14, 2023
- |
- Full Page
The U.S. Food and Drug Administration on Friday approved the first nonhormonal medication aimed at easing menopause hot flashes.
The new pill, called Veozah (fezolinetant), is from a class of drugs called neurokinin 3 (NK3) receptor antagonists. It targets a specific brain neuron that's thought to be set off balance as estrogen levels naturally decline during menopause.
"It works b...
- Ernie Mundell HealthDay Reporter
- |
- May 13, 2023
- |
- Full Page
Outside advisors for the U.S. Food and Drug Administration voted Thursday to recommend approval of Neffy, the first epinephrine nasal spray for severe allergic reactions.
Although most of the Pulmonary-Allergy Drugs Advisory Committee members supported the spray for adults (16:6) and children (17:5), key questions linger about whether more data is needed from its maker, ARS Pharmaceutical...
- Cara Murez HealthDay Reporter
- |
- May 12, 2023
- |
- Full Page